Last week, we outlined the basics of a Safe Quality Food (SQF) certification, examining the benefits, costs and the steps. However, the biggest piece of this food safety certification is passing the actual audit. Here, we provide a comprehensive look at the initial SQF certification audit process, including the time frame and scoring parameters.
After reading our previous post, you already know that you must complete the following steps prior to your SQF audit:
- Learn the SQF Code
- Select the relevant SQF modules
- Register your company with SQF’s Assessment Database
- Designate an employee as the “SQF Practitioner”
- Choose your certification level
- Obtain proposals from SQF-licensed certification bodies
- Conduct a pre-assessment (optional)
- Choose a certification body and schedule an audit
However, you before the audit occurs, you must also ensure you identify your scope of certification. It is important to note that the supplier and certification body must agree on the level of certification the supplier wants before the audit begins. Once this is decided, it cannot be changed.
Remember, SQF certification is site and product specific, and the scope of certification forms part of the certificate. It describes:
- The site
- Food sector categories
- Products processed
- Product handled
To learn more about the scope, refer to the SQF Code.
2 stages of the SQF audit
Your SQF audit involves two stages:
- Desk audit—This stage verifies that your SQF System documentation meets the requirements of the SQF Code. Note that desk audits are not scored. Your desk audit ensures:
- The designation of a qualified SQF practitioner
- The food safety plan (at level 2) and the associated Critical Control Point (CCP) determinations, validations and verifications are appropriately documented and endorsed by your SQF practitioner
- The food quality plan (at level 3) and associated Critical Quality Point (CQP) determinations, validations and verifications are appropriately documented and endorsed by your SQF practitioner
- The documented system is relevant to the scope of certification and the products processed there under
- Facility audit—This is conducted on site at an agreed-upon time between you and your certification body (when your facility’s main operations are running). The facility audit determines how effectively you’ve implemented the SQF System and involves a review of your entire facility, including the inside and outside of the building, regardless of the scope of certification. It also establishes and verifies:
- Food safety hazards (level 2) and food quality hazards (level 3) are effectively identified and controlled
- Effective interaction between all elements of the SQF System
- Your demonstrated level of commitment to maintaining an effective SQF System/ meeting the food safety regulatory and customer requirements
Mandatory SQF system elements
The following elements are considered mandatory and will be evaluated during your SQF audit:
- Management Policy
- Management Responsibility
- Food Safety and Quality Management System
- Management Review
- Document Control
- Records
- Food Legislation
- Food Safety Fundamentals
- Food Safety Plan (at level 2, 3)
- Food Quality Plan (at level 3)
- Product Release
- Validation and Effectiveness
- Verification and Monitoring
- Corrective and Preventative Action
- Internal Audit
- Product Identification
- Product Trace
- Product Withdrawal and Recall
- Food Defense
- Training Program
The SQF audit time frame
The below tables outline how long each stage of the audit is expected to take.
Desk Audit Duration Table
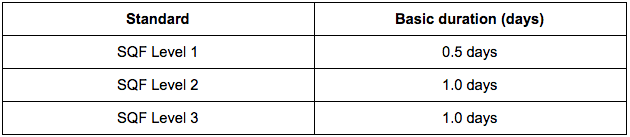
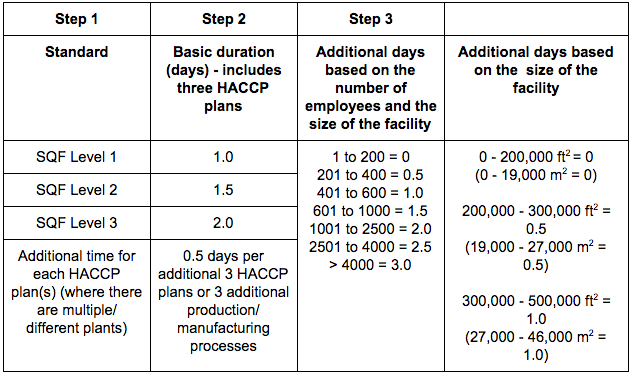
Facility Audit Duration Table
What happens when you do not meet the SQF standards
Your audit is scored based on how well you meet the SQF Code. In places where you do not accurately meet these standards, the auditor records those areas as non-conformities, or non-conformances. There are three types of non-conformities:
- Minor—If minor non-conformities are fixed within 30 days of the facility audit, then they are “closed out.”
- Major—Major non-conformities should be corrected within 14 days of your facility audit.
- Critical—A critical non-conformity will result in a failed facility audit.
If the auditor finds room for improvement in your food plant that doesn’t qualify as a non-conformance, these areas are recognized by the auditor as not being an industry best practice. These do not require a corrective action response like the non-conformities. Instead, these notes are given to communicate what ways your plant could improved re: following SQF guidelines.
Scorings and ratings
Facility audit scores are determined on a scale of 0-100. Minor, major and critical non-conformities all result in point loss.

If you receive an “F – Fails to comply,” you will not receive a certificate and must re-apply for another facility audit.
Maintaining your SQF certification
Your plant will remain SQF-certified for 75 days after the anniversary of your audit date.
To maintain certification, you must receive at least a “C – Complies” at re-certification audits within the required time frame. If you had non-conformities that were reported on an audit, it is up to you to correct these discrepancies within the window provided by your certification body.
If you’d like to learn more about the SQF certification audit process, visit the SQFI website or email me at foodforthought@stellar.net.


