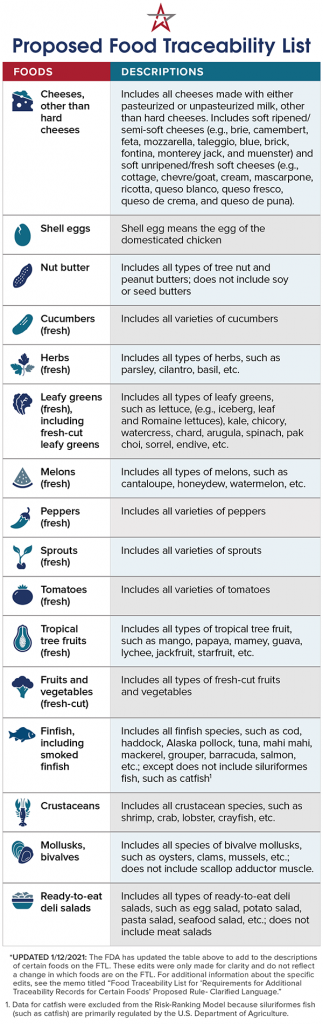
The U.S. Food and Drug Administration (FDA) is proposing additional traceability recordkeeping requirements for those who manufacture, process, pack or store foods included on the Agency’s new Food Traceability List. The proposed rule is a key component of its New Era of Smarter Food Safety Blueprint and would implement Section 204(d) of the Food Safety Modernization Act (FSMA).
The additional recordkeeping requirements would apply not only to foods specifically listed on the Food Traceability List, but also to products that contain these foods as ingredients. Let’s look at what’s included:
According to the proposal, parties who produce, process, pack and store these foods would need to establish and maintain records containing so-called “Key Data Elements” (KDEs) associated with different “Critical Tracking Events” (CTEs).
Records containing KDEs would be required for the following CTEs along the supply chain:
- Growing
- Receiving
- Transforming
- Creating
- Shipping
The records required at each CTE would need to contain and link the traceability lot code of the food to the relevant KDEs.
Additionally, the proposal would require those subject to the rule to establish and maintain traceability program records. These records are intended to help regulators understand an entity’s traceability program, and include:
- A description of relevant reference records
- A list of foods on the Food Traceability List that are shipped
- A description of how traceability lot codes are assigned
- Other information needed to understand data provided within the required records
According to the FDA’s proposal, any final rule on the additional traceability requirements would become effective 60 days after it’s published in the Federal Register.
“Because an effective traceability system requires all entities in a supply chain to maintain traceability records, we believe all persons subject to the rule should come into compliance by the same date,” notes the Agency. “We propose that the compliance date for all persons subject to the recordkeeping requirements would be 2 years after the effective date of the final regulation.”
The effective date for the proposal is still pending.
So what does this mean for food industry players moving forward? If your facility still manages traceability manually — rather than collecting data via an automated manufacturing execution system (MES) — it could spell more risk.
But rather than viewing the FDA’s proposal as just another regulatory hoop to jump through, think of it as an opportunity: Modernizing your traceability program can actually yield tangible benefits for your business and bottom line. Stay tuned for my next post where we’ll spell out what those benefits are and how you can take advantage of them.
Still have questions about the proposed changes? Check out these Frequently Asked Questions compiled by the FDA. Want to connect with us for more information on food safety compliance and traceability? Email me at foodforthought@stellar.net or give us a call at 904.260.2900.